This page has been automatically translated. Please refer to the page in French if needed.
Drug reimbursement
First treatment for vitiligo under the Health Insurance program
Publié le 02 février 2024 - Directorate for Legal and Administrative Information (Prime Minister)
An order of 26 January 2024 authorizes the placing on the market of a cream for patients with vitiligo. This new treatment is 100% supported for adults and adolescents over 12 years of age.
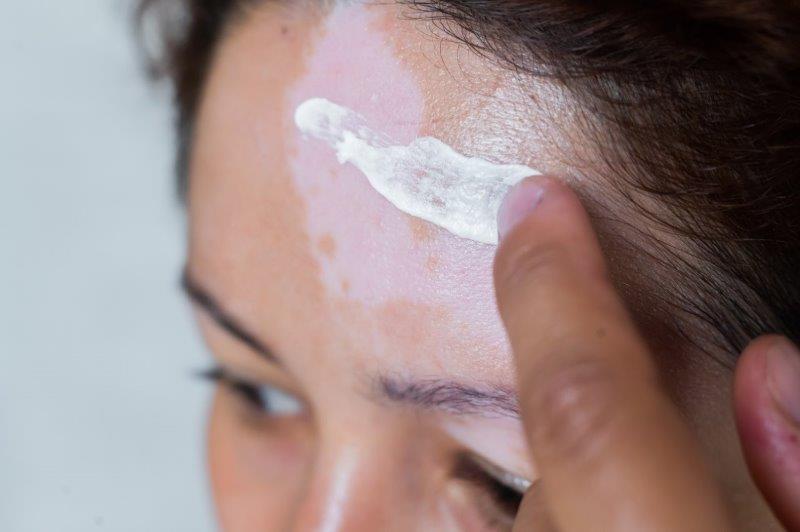
Vitiligo is an autoimmune disease that causes areas of the skin to depigment, due to the disappearance of certain cells called melanocytes.
In France, no treatment was available to treat vitiligo. An order of 26 January 2024, published in Official Journal of 31 January 2024 authorizes the sale and the full reimbursement of a first treatment.
The treatment, a cream to be applied to the skin, is deliverable only in hospital pharmacy, in certain establishments. It's available on ordinance.
This authorization was granted to the cream Opzelura from the Incyte Biosciences laboratory.
The management of this cream concerns adults and adolescents over 12 years of age for “treatment of non-segmental vitiligo with facial involvement”, as specified in the order of 26 January 2024.
FYI
this new treatment was granted on april 19, 2023 a marketing authorization valid throughout the European Union. In France, it is put into circulation as part of the direct access scheme adopted by the Social Security Financing Act 2022. Dispensing of the medicinal product is possible after publication of the favorable opinion of the High Health Authority.
Additional topics
Service-Public.fr
Service-Public.fr
Service-Public.fr
Service-Public.fr
High Health Authority (HAS)
Agenda
Du 14 avr. au 15 juin 2025
Prévention Covid-19
Publié le 02 avril 2025
À partir du 2 avr. 2025
ETA
Publié le 24 mars 2025

